Technical Requirements for Isolators and RABS Used in Aseptic Manufacture
News & Insights2023-02-16
Introduction
We all know that the new draft of Annex 1 certainly suggested that Isolators are probably the best way forward when dealing with Sterile/Aseptic manufacture. In reality, this is not new the FDA has long stated that they will look more closely at businesses that do not adopt this approach and it is suggested in the ISPE Guide to Sterile Production. So, it is hardly a new concept, but perhaps Annex 1 puts more emphasis on this now mature isolator and RABS-based methods of production.

So, what does this all mean for the current users of this technology and those looking to switch over to its use on existing processes or looking to buy equipment either integrating contained/isolated systems or purchasing fully contained/isolated systems or processes?
Section 4 Barrier Technologies of EU GMP Annex 1 Manufacture of Sterile Medicinal Products suggests that requirements for the design, background environment, the frequency of glove replacement, decontamination method, etc. should all receive attention, and as one of the businesses supplying this equipment, we have been doing this for many years for our customers.

Annex 1 now formalizes this approach which would suggest that the inspection authorities will expect to see considered in any design.
So, what exactly do you need to do or understand as a customer or with regards to the containment or isolation of your process?
What is the definition of an isolator according to the current guidance?
1.Isolator
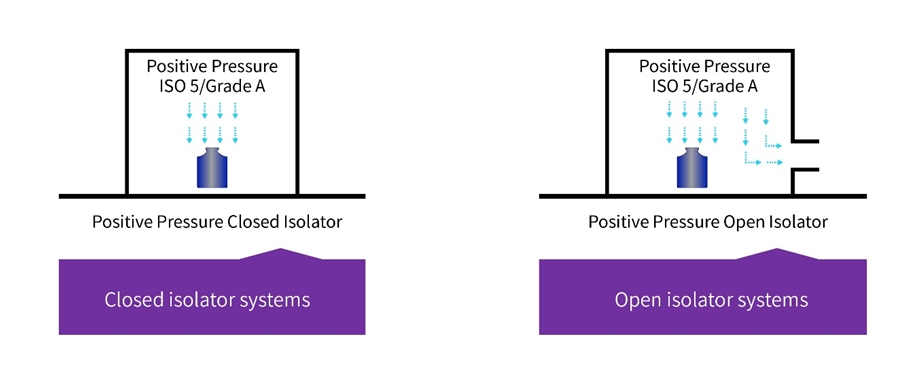
Figure 1 – Types of isolators3
▪ In simple terms, a closed isolator system is defined as something that excludes external contamination of the interior of the isolator by achieving material transfer using an aseptic connection to auxiliary and/or secondary equipment, rather than the use of openings to the surrounding environment. Therefore, closed systems remain sealed throughout operations.
This is in general how most businesses see isolators for sterile processes, certainly for Grade D and above.
▪ However, there are some instances where open isolator systems are used this design allows for the continuous or semi-continuous ingress and/or egress of materials during operations through one or more openings. Openings are engineered (e.g. using continuous overpressure) to exclude the entry of external containment into the isolator.
▪ All the above will use HEPA filtration generally Laminar flow, but if the airflow is managed correctly turbulent flow can be used, provided air studies demonstrate that there are no dead spots inside the isolator.
2.RABS (Restricted Access Barrier System)
▪ (RABS), something from a personal perspective I have never felt comfortable using RABS in sterile production, as the system is enclosed, but not fully sealed, with the environment meeting defined air quality conditions (for aseptic processing grade A). But not being fully enclosed can lead to issues. However, in general, they are manufactured as a rigid-wall enclosure (Generally a Glass and 316 Stainless-Steel structure) and use integrated gloves/gauntlets to separate the RABS interior from the surrounding cleanroom environment.
The inner surfaces of the RABS are disinfected and decontaminated using sporicidal agents. Operators can use gloves ports, half suits, RTPs and other integrated transfer ports to perform operations such as removing line blockages, in-process tests, and other manipulations and/or conveying materials into the operational interior of the RABS. The normal practice would be to ensure that the doors are rarely opened, and only under strictly pre-defined conditions.
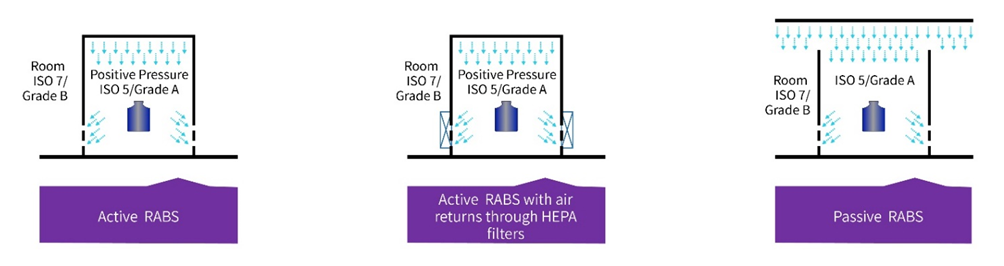
Figure 2 – Types of RABS3
▪ Active RABS use an air overspill and overpressure into the room to maintain the grade inside the RABS the air supply inside the RABS, as discussed previously uses integral HEPA filters air supply which will provide UAF to the critical process with the excess air overspill leaving the RABS below the critical process.
▪ Active RABS with air returns through HEPA filters: These RABS, which are very close to a fully isolated system use air returns through HEPA filters with integral HEPA-filtered air supply providing UAF to the critical process. They also use air return through HEPA filters to prevent hazardous products migrating into the surrounding area or microbial contamination from migrating into the RABS.

▪Passive RABS with air overspill to the surrounding: Passive RABS use air overspill to the surrounding, the airflow to the critical area is provided by ceiling-mounted HEPA filters and the bottom of the enclosure is open to the room to provide for airflow through the system, these are processes that really require a lot of onsite management as there is little room for error and significant risk of contamination.
My general comment is even if a RABS is operating correctly the user must pay particular attention to the equipment inside the containment system, of whatever type, as quite often, if the equipment inside the protective isolator/RABS envelope is engineered badly, it can become the biggest risk. Things to consider with any equipment inside an isolator can include whether it can be cleaned, can it be sterilized, is it easy to assemble and disassemble, whether can it be accessed via the manipulations system supplied, can samples be taken easily both in-process and microbiological samples and whether has it been ergonomically assessed. None of this is easy to achieve it should form part of the design study/risk assessment at the outset. The best-designed RABS or Isolators are only as good as the equipment supplied inside, so this must not be forgotten.
Technical requirements
Based on everything that has been discussed above I have tried to look at the technical requirements that should be considered for both RABS and isolators used in aseptic manufacture, these are both regulatory considerations and operational considerations, as ever it is not an exhaustive list but should help guide you on your journey into this technology. Certainly, I would expect to see many of these points written into any URS that any potential supplier would receive once you have decided on the RABS or Isolator type you need for your process. Again, this should not be driven only by Annex 1, it really should be built into any operational system, Annex 1 in my view really articulates, from a regulatory perspective what any good business should be doing anyway, certainly any of us in the containment industry would have already taken much of this onboard prior to Annex 1 being issued in its current form:
Written by
Ewart Richardson AUSTAR Consultant
info@austar.com.hk
https://www.austarunion.com/
References:
[1]EC EudraLex - Volume 4 GMP guidelines Annex 1 Manufacture 1 of Sterile Products, 2022
[2]EC EudraLex - Volume 4 GMP guidelines Annex 1 Manufacture 1 of Sterile Products V.13, 2021
[3]ISPE Baseline® Pharmaceutical Engineering Guide, Volume 3 –Sterile Product Manufacturing Facilities, International Society for Pharmaceutical Engineering (ISPE), Third Edition, 2018, www.ispe.org.
[4]PDA TR34 Design and Validation of Isolator Systems for the Manufacturing and Testing of Health Care Products, 2001







 Search
Search 中文
中文











