Influence of Stoppers on Residual Moisture Content during Storage of Freeze-dried Drug in Vial
News & Insights2023-08-11
The residual moisture content is one of the most important critical quality attributes of a freeze-dried product. It has significant impact on the product quality during storage. While the freeze-drying cycle has the most profound influence on the residual moisture content of the final product, primary packaging material, particularly the vial stopper could also affect the residual moisture content of a freeze-dried product during storage.

The stoppers for vials contain a certain amount of water, which depends on the composition of the stoppers. De Grazio and Flynn1 showed that the selection of the polymer, the additives for the vulcanization, and the filler influence the adsorption and desorption of water. However, even the best possible mixture increases the residual moisture in 215 mg of sucrose from 1.95 to 2.65% during 3 months of storage at room temperature. Other stopper mixtures show an increase of up to 1.7%. Pikal and Shah2 demonstrated that the desorption of water from the stopper and the absorption of water by the product depend, in the equilibrium state, on the mass and water content of the stopper and the water content and sorption behavior of the dry product.
If the stopper is as small as technically possible and its material optimized, the water content of the stopper depends on its prehistory: Steam-sterilized stoppers take up water (e.g. 1.1% of their weight), which can only be removed by 8 hours of vacuum drying2 or by 8 hours of recirculated hot air (110°C) drying down to 0.1%3. Figure 12 shows that a steam-sterilized stopper, vacuum dried for 8 hours, releases slightly less water to lactose than does an untreated stopper. A stopper dried for only 1 hour increases the residual moisture in 6 months of storage at 25°C by a factor of 2.4. Figure 1 also shows that an equilibrium is reached that, practically, does not change later. The time to reach the equilibrium depends strongly on the temperature. For a given product, the time to reach half-maximum increases from 4 days at +40°C to 10 months at +5°C. It is surprising that the absorption isotherms for lactose are found to be independent of temperature at +25°C and +60°C; this applies also to vancomycin at +25°C and +40°C. Figure 2 shows the equilibrium water content as a function of stopper treatment and the amount of dry product independent of the storage temperatures of +25°C and +40°C for two different products; vancomycin is clearly more hygroscopic than lactose.
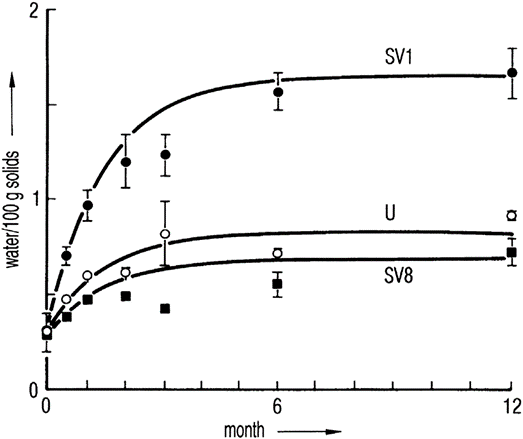
Figure 1 Water content of 100 mg of lactose at +25°C as a function of time. The vials were closed with 13 mm stoppers subjected to different pretreatment: SV1, steam-sterilized; U, untreated; SV8, steam-sterilized followed by vacuum-drying for a minimum of 8 h. The lines were calculated by a model system.
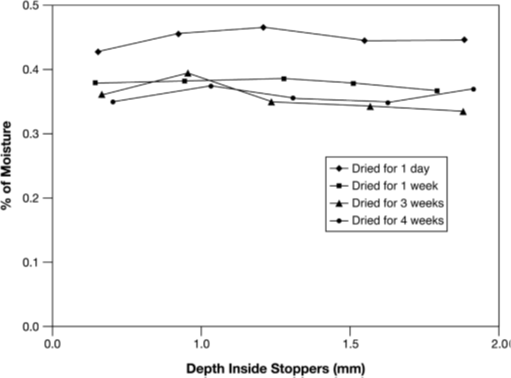
Figure 2 Equilibrium water content in two different freeze-dried products, each with two different amounts of product per vial. The equilibrium data are extrapolated from the +25°C and +40°C values. SV1, U, and SV8 as in Figure 2; 1, 25 mg of lactose; 2, 100 mg of lactose; 3, 25 mg of vancomycin; 4, 100 mg of vancomycin.
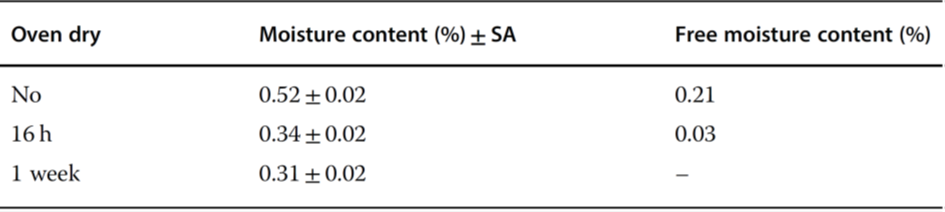
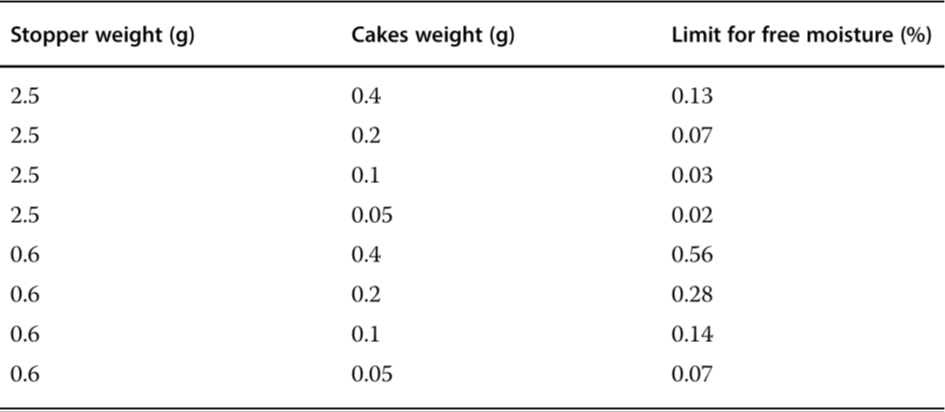
Earle et al.4 showed that the residual moisture in the product Pedvax HIB TM did not change during storage at 2 – 8°C for 24 months if the stoppers were steam-sterilized, vacuum-dried for 6 hours, and finally dried at +143°C for 4 hours. If the vials were closed with stoppers that had not been dried, the residual moisture increased in 12 months to ~5.3%. Danielson5 warned against toxic components that could diffuse or migrate from the stoppers to the product. A protective coating does not prevent the extraction of these substances, but a Teflon coating is better than none. Corveleyn et al.6 determined the water content of five chlorobutyl and three bromobutyl stoppers in the range of 0.85 – 1.49 and 1.71 – 1.99%, respectively, after they had been stored for 85 days at 95% RH. During sterilization, the moisture uptake was 0.82 – 0.9% for the chlorobutyl and 0.41 – 0.57% for the bromobutyl stoppers. Wang et al.7 differentiated between free and bound water in stoppers. Figure 3 shows the moisture distribution in stoppers (FM257/2, V9032, Helvoet Pharma, Pennsauken, NJ, USA) after autoclaving at 121°C for 30 minutes and drying at 100°C for different times. The moisture distribution in Figure 3 no longer changes after 1 week of drying. The remaining amount of ~0.35%, bound to the stopper material, cannot be removed at 100°C. The authors concluded that water that cannot be removed at 100°C is bound in such a way that it cannot jeopardize the pharmaceutical product. Only the free water can diffuse from the stopper to the product. The moisture content is measured by the Karl Fischer method with different temperatures in the oven, 100°C to determine the free water content and up to 300°C to measure the free water and bound water. The author suggested developing a similar program for other stoppers, since the time for such measurement is relatively short (1 week) instead of observing the residual moisture in a product over long times. Table 17 summarizes the results with the stoppers described above. Table 27 lists the limits of the free moisture content in two types of stoppers and for different cake weights under the assumption that maximum residual moisture increase of 0.5% is acceptable.
Figure 3 Moisture distribution inside the described rubber stoppers that were autoclaved at 121°C for 30 nub and then dried at 100°C for different times.
Table 1 Moisture analysis of FM257/2, V9032 stoppers (Helvoet Pharma, Pennsauken, NJ, USA)
All stoppers were autoclaved for 16 minutes, vacuum dried for 30 minutes, and oven dried for the specified time.
Table 2 Maximum moisture content of stoppers for a hypothetical product with a 0.5% moisture increase limit.
In conclusion, to achieve optimal quality and stability of a freeze-dried product, one should also pay attention to the treatment of vial stoppers, which could considerably impact the residual moisture content of dried product, which in turn, affects the stability of the product during storage.
Reference:
1.DeGrazio, F. and Flynn, K. (1992) Lyophilization closures for protein based drugs. J. Parenter. Sci. Technol., 46, 54–61.
2.Pikal, M.J. and Shah, S. (1992) Moisture transfer from stopper to product and resulting stability implications, in Developments in Biological Standardization, vol. 74 (eds J.C. May and F. Brown), Karger, Basel, pp. 165–179.
3.Brinkhoff, O. (1993) Primärpackmittel für Lyophilisate, in Lyophilisation, vol. 35 (eds D. Essig and R. Oschmann), Wissenschaftliche Verlagsgesellschaft, Stuttgart, p. 145.
4.Earle, J.P., Bennett, P.S., Larson,K.A., and Shaw, R. (1992) The effects of stopper drying on moisture levels of haemophilus influenzae conjugate vaccine, in Developments in Biological Standardization, vol. 74 (eds J.C. May and F. Brown), Karger, Basel, pp. 203–210.
5.Danielson, J.W. (1992) Toxicity potential of compounds found in parenreral solutions with rubber stoppers. J. Parenter. Sci. Technol., 46, 43–47.
6.Corveleyn, S., De Smedt, S., and Remon, J.P. (1997) Moisture absorption and desorption of different rubber lyophilization closures. Int. J. Pharm., 159, 57–65.
7.Wang, Z., Frankel, B.A., and Lambert, W. (2001) Determination of moisture in rubber stoppers: effect of Karl Fischer oven temperatures. PDA J. Pharm. Sci. Technol., 55, 162–170.







 Search
Search 中文
中文










