Overcoming Common Issues during Lyophilization
News & Insights2023-10-11
During the freeze-drying process, some unexpected or undesirable events could occur , resulting in serious failures of the process. Some of the issues are listed and discussed below.

1.Prolonged Evacuation Time
The evacuation of the plant takes longer than calculated from the volume and the pumping capacity, in spite of regular leak checks. The event is most likely related to the formation of ice, which is condensed during loading on the precooled shelves. The extent of this depends on the shelf temperature during loading and the moisture content of the gas in the chamber. One of the possible courses of actions could be as follows: Check the condenser temperature in relation to the pressure reached. If the condenser temperature is, for example, -42 °C, Ps (saturation vapor pressure) 0.1 mbar, but the lowest pressure reached in the chamber is only ∼0.5 mbar, then the condenser capacity is not the bottleneck, but the slow sublimation of some ice. Raising Tsh (shelf temperature) from, for example, -40 °C could be started very slowly in such a way that the pressure in the chamber does not exceed p s of the maximum tolerable Tice (temperature of ice at the sublimation front), for example, -22 °C (Ps 0.85 mbar). This may take one or more hours, depending on the heat transfer to the undesired ice. When all excess ice has been removed, the pressure will drop quickly to the value close to p s of the ice on the condenser and the normal cycle can be started. If, in another example, the condenser temperature is the limiting factor, then the condenser is at capacity limit and one has to delay the heating until the condenser temperature falls. All this can be avoided if the door of the chamber is only partially opened during loading and a low pressure of dry gas is kept in the chamber.
2.Slow Pressure Increase in the Chamber during Main Drying
In spite of a constant or decreasing condenser temperature, the pressure in the chamber rises after main drying has started correctly. This may be due to the development of a leak, although this is unlikely in a plant leak-tested before the run, and a newly developed leak may alter the pressure slightly. In addition, a leak that worsens constantly during the run is possible, although unlikely. Among other reasons, rising pressure may occur due to a constant increase in the pressure of permanent gases. This will increasingly hinder transport of water vapor and reduce the condensing effectiveness of the condenser. The increase may occur because of two reasons: After the start of main drying, air dissolved or included in the frozen ice is freed as the ice is sublimed. The amount of included gas can vary by a factor of almost 100, depending on the material used and its fabrication history. If the capacity of the vacuum pump is smaller than the amount of freed gas at the desired pressure, the condenser is increasingly filled by permanent gases. The other possibility is a misplaced suction pipe of the vacuum pump at the condenser. The common permanent gases have a higher density than water vapor. If the vacuum connection is not in the lowest area of the condenser, the gas will slowly fill the condenser chamber from the bottom up to the suction level of the pump, thus reducing the condenser efficiency to a greater or lesser extent.
3.Stoppers “Pop Out” or Slide into the Vials
The "pop out" of stoppers occurs mostly during evacuation. If the product is not completely frozen and contains highly concentrated, but not solidified, inclusions, its water content may evaporate explosively when the vacuum is applied. This abrupt evaporation will also blow some product particles to the walls of the vial, as can be seen in the vials after drying.
Stoppers may also slide into the vials and cause their virtual closure. This can happen during the freezing of the product, especially if the shelf temperatures are very low. The dimensions of the stoppers need to be tested not only for the pressure to close them after drying, but also for shrinking at low temperatures.
4.Trace of Highly Volatile Solvents (Acetone, Ethanol)
The presence of such traces always requires some special steps, depending on the amount of the volatile component. If the traces cannot be removed before freezing and drying, they can (i) influence the structure during freezing, (ii) disturb the condensation of ice, and (iii) contaminate the oil in the vacuum pump. As shown in Figure 1, the vapor pressures of ethanol and acetone at -70 °C are approximately 2 × 10-2 and 3 × 10-1 mbar, respectively (ice 3 × 10-3 mbar). Ethanol melts at -114 °C and acetone at -95.5 °C. If the amounts are small, a very quick freezing might help to distribute the solvents very uniformly in the ice. If the amounts are large enough, they will form a veil on the condenser surface, disturbing the efficiency of the condenser, drip as a liquid from the condenser surface and evaporate from the warmer condenser wall. Furthermore, gauges with hot wires (thermal conductivity gauges) should not be used and finally the oil of the vacuum pumps must be recycled and cleaned or it will be contaminated by the solvents and the pumps will no longer discharge the solvent vapors. These problems may be reduced by placing an LN2 -cooled trap between the condenser and vacuum pump set. Of course, some ice will also condense in the trap, but this amount will be small, as the water vapor pressure of ice in the condenser is very low compared with the vapor pressure of the solvent at the condenser temperature. However, this trap solves the problems in the condenser, mentioned before, only if it is designed also for this task. A substantial effort is justified to remove such solvents before freezing starts.
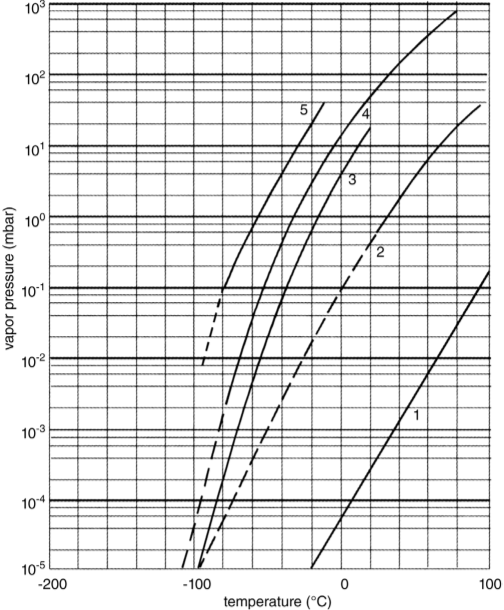
Figure 1 Vapor pressures of solvents as a function of temperature. 1: glycerin; 2: dimethyl sulfoxide (C3H8O3); 3: water; 4: ethanol; 5: acetone.
5.Different Structures of the Dried Product in the Center and Border of a Shelf
The outer vials are influenced (if the shelf temperature is uniform) by a different temperature of the walls and the door of the chamber. If the chamber walls and the door are not kept at shelf temperature, the outer vials must be shielded or they may be too warm during freezing (e.g., freezing differently) or too cold during secondary drying and this may lead to a different residual moisture content from that in inner vials.
Clearly, the issues discussed above are far from complete. If you encounter any issues during the freeze-drying process, experienced engineers and scientists from AUSTAR are glad to help you identify the course of the issue and provide solutions.







 Search
Search 中文
中文







