Enhancing Operational Safety: Robust Containment Solutions for OSD Processes
News & Insights2024-04-10
In recent years, there has been a significant uptick in the production of highly potent drugs in the Oral Solid Dosage (OSD) sector. Consequently, the emphasis on containment measures has grown exponentially within the industry. Working with such potent compounds mandates stringent containment protocols to mitigate the risks associated with potential material leaks and inadequate cleaning practices, which could have dire consequences. Furthermore, tightening regulations and the increasing complexity of manufacturing processes underscore the imperative for top-tier containment solutions to ensure compliance and operational integrity.

Understanding Highly Active Substance (Potent Compounds)
A highly active or potent substance refers to a chemical or pharmaceutical compound that is effective at low concentrations. These substances often have a strong biological or therapeutic effect even in small amounts. In the context of pharmaceuticals, highly potent drugs are designed to be effective in low doses, which can be critical for minimizing side effects and improving the overall safety and efficacy of the medication. Handling and manufacturing such substances require special precautions due to their potency and potential for significant impact at low concentrations.
lA common classification of potent compounds based on potency:
Highly Potent Compounds: These compounds exhibit significant pharmacological activity at very low doses. They are often effective in concentrations of micrograms or even nanograms per kilogram of body weight. Examples include certain chemotherapy drugs, potent painkillers, and some hormone therapies.
Moderately Potent Compounds: These compounds have a moderate level of pharmacological activity and typically require higher doses to achieve therapeutic effects than highly potent compounds. Examples include some antibiotics, antihypertensive drugs, and antidepressants.
Low Potency Compounds: These compounds have relatively low pharmacological activity and require higher doses to produce desired effects. They are generally considered less potent compared to highly or moderately potent compounds. Examples include some over-the-counter pain relievers, vitamins, and certain antacids.
It's important to note that the classification of a compound as potent can vary depending on the specific context, such as its intended use, the target population, and regulatory considerations.
lClassification of the compound as highly potent:
Occupational Exposure Limit (OEL) of ≤ 10 μg/m3:
ØThis indicates the maximum concentration of the substance allowed in the air of a workplace to which workers can be exposed over a specific period without adverse health effects.
ØIn this case, the occupational exposure limit is set at 10 micrograms per cubic meter (μg/m3), emphasizing the need for strict control of airborne concentrations to protect workers.
Daily Therapeutic Dose of ≤ 10 mg/day:
ØThis represents the recommended or prescribed amount of the substance that can be safely administered to a person within 24 hours to achieve the intended therapeutic effect.
ØThe daily therapeutic dose is restricted to 10 milligrams (mg) or less, suggesting that the substance is potent and effective even at relatively low doses.
Biological Activity at 150 μg/kg Body Mass in Humans:
ØThis indicates the level at which the substance exhibits biological effects within the human body based on body mass.
ØThe biological activity is observed at 150 micrograms per kilogram (μg/kg) of body mass, highlighting the potency of the substance and its ability to elicit a physiological response at a relatively low concentration in the body.
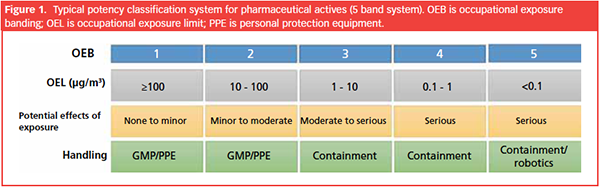
Operator Protection via PPE for Different OEB Levels:
lOEB 1 [Non-Toxic]:
ØPPE requirements for OEB 1 substances are typically minimal.
ØOperators may need basic PPE such as lab coats, safety glasses, and disposable gloves.
ØStandard hygiene practices, including handwashing and avoiding contact with eyes or mouth, are usually sufficient.
lOEB 2[Almost Non-toxic or Very less potent]:
ØPPE requirements become more stringent for OEB 2 substances due to very low potency and associated risks.
ØOperators may require enhanced PPE, including disposable coveralls (cGMP Gowning), respiratory protection (such as N95 masks), and chemical-resistant gloves.
ØAdditional precautions might include the use of protective face shields or goggles when handling potentially aerosolized or splashing substances.
lOEB 3 [Moderately Toxic]:
ØOEB 3 substances are moderately potent and present significant risks to operators.
ØComprehensive PPE is essential, including full-body protective suits, double-gloving with chemically resistant gloves.
ØSpecialized containment equipment such as isolators or glove boxes may also be necessary to minimize exposure.
ØStrict decontamination procedures should be implemented, including dedicated changing areas and shower facilities.
lOEB 4 & OEB 5:
ØOEB 4 & OEB 5 substances are extremely potent and pose severe health risks.
ØMaximum protection is required, with PPE including fully encapsulated chemical protective suits, high-level respiratory protection (e.g., self-contained breathing apparatus), and rigorous adherence to stringent containment protocols.
ØUtilizing equipment with advanced containment features and employing closed systems for material handling
ØPersonnel handling OEB 4 /OEB 5 compounds may need specialized training and periodic medical surveillance to monitor for potential exposure-related health effects.
Unlocking Safety: Tailored Containment Solutions Across OEB Levels
lDownflow Booth
Downflow booths are enclosed, ventilated workstations designed to control airborne contaminants generated during processes such as powder handling, weighing, and mixing. They typically utilize a downward airflow to capture and contain particles, preventing their dispersion into the surrounding environment.


OEB3: Achieving OEB 3 containment levels is a common application for downflow booths
§Utilizing engineered controls that secure containment through air entrainment
§The unidirectional airflow effectively captures and prevents the dispersion of dust particles into the air.
OEB 4/OEB 5: OEB 4/OEB 5 containment levels is a common application for downflow booths
The addition of a containment screen enhances the effectiveness of the downflow booth in containing highly potent substances. The containment screen acts as an additional barrier, intercepting any particles that may escape the primary airflow pathway. This secondary containment measure provides an extra layer of protection, reducing the risk of operator exposure and ensuring compliance with stringent containment requirements for OEB 4 substances.
lIsolators [ Rigid or Hard/Soft or Flexible]
Rigid or hard isolators are advanced containment systems designed to provide a secure and controlled environment for handling hazardous materials or processes. Their robust construction, sealed design, controlled airflow, and operator interface features make them indispensable tools for ensuring safety, protecting personnel, and maintaining product integrity in critical applications.


Rigid isolators are typically constructed as a sealed enclosure with solid walls and airtight seals to ensure containment integrity. The construction materials are selected for their durability, chemical resistance, and ease of decontamination. Rigid isolators achieve containment through a combination of physical barriers, sealed entry points, and controlled airflow. The interior of the isolator is maintained at a higher pressure than the surrounding environment, preventing the escape of contaminants.
Glove ports are affixed to the window, aligning with glove sleeves for operator interaction. The isolator also includes both inlet and outlet HEPA filters, accompanied by positive or negative blowers. Additionally, utility panels and specialized ports, such as Rapid Transfer Ports (RTP), can be incorporated for the seamless introduction or removal of items into or from the isolator.
Soft or Flexible Isolators are characterized by their flexible construction, portability, and adaptability, making them ideal for a variety of applications where containment needs may vary.
Soft isolators are constructed from flexible, impermeable materials such as polyethylene or polyurethane. These materials allow for easy assembly, disassembly, and reconfiguration of the enclosure. Soft isolators typically feature welded seams, zipper closures, or other secure fastening mechanisms to maintain containment integrity.
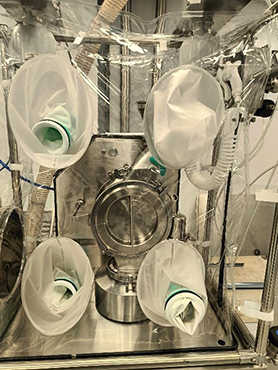

Operators interact with the contents of soft isolators through integrated glove sleeves or ports, which allow them to manipulate equipment, perform tasks, or handle materials inside the enclosure while maintaining a physical barrier between themselves and the contained substances. They are lightweight, portable and are ideal for use in settings where space is limited or where temporary containment solutions are needed.
lSplit Butterfly Valves
Split butterfly valves can be effectively employed in OEB 4 and OEB 5 applications to facilitate the safe transfer of highly potent materials between containment zones and preventing the release of hazardous substances.
Its design consist of two halves, each with a circular disc or butterfly plate mounted on a central shaft. The valve is divided along its vertical axis, with each half connected to a separate containment chamber or process line. The two halves of the valve are joined by a central flange or connection point. It is designed to provide a secure seal when closed, isolating the two containment zones. When the valve is in the closed position, the two halves of the butterfly plate meet at the centerline, forming a complete seal that prevents the escape of materials. To open the valve, the two halves of the butterfly plate rotate outward simultaneously, creating an unobstructed pathway for material transfer between the two chambers.


lContinuous Liners or Continuous Liner Bags
Continuous liners, also known as continuous liner systems or continuous liner bags, are specialized containment solutions used in pharmaceutical manufacturing to facilitate the transfer of materials while maintaining containment and preventing contamination.


It consist of a flexible, impermeable bag or liner that is connected to a dispensing or processing vessel. The liner is typically made from materials such as polyethylene or polypropylene and is designed to fit snugly inside the vessel, forming a barrier between the vessel's contents and the external environment.
It is used during material transfer processes to prevent cross-contamination and ensure the integrity of the product. The liner is filled with the material to be transferred, either manually or automatically, and then sealed to prevent leakage or spillage. Once filled, the liner can be removed from the vessel and transported to the next stage of the process.
Some liners are designed to be disposable and are discarded after a single use, while others are reusable and can be cleaned and sterilized for repeated use. They are commonly used in pharmaceutical manufacturing for transferring powders, granules, and other materials between process equipment while maintaining strict containment.
lGlove Port Tester
A glove port tester is a specialized tool used to verify the integrity and effectiveness of glove ports in containment systems such as gloveboxes, isolators, or glove bags. These systems are commonly used in pharmaceutical manufacturing to provide a barrier between operators and potentially hazardous materials.


During testing, the glove port tester is attached to the glove port, and a pressure differential is created between the inside and outside of the enclosure. This pressure difference simulates the conditions that the glove port would experience during normal operation. The tester then measures any airflow or leakage around the glove port, indicating the effectiveness of the seal.
Regular testing of glove ports with a glove port tester helps maintain the safety of operators working with hazardous materials and ensures the effectiveness of containment systems in preventing exposure to contaminants.
Application of Containment Solutions in OSD Manufacturing Plant
Containment solutions play a crucial role in ensuring the safety of personnel and the integrity of products in Oral Solid Dosage (OSD) equipment used in pharmaceutical manufacturing.
lContainment Solutions on OSD Equipment
Weighing and Dispensing Isolator:

Integrated Granulation Suite [Closed material transfer using SB Valves]

Material Handling Tablet Press


Tablet Coater
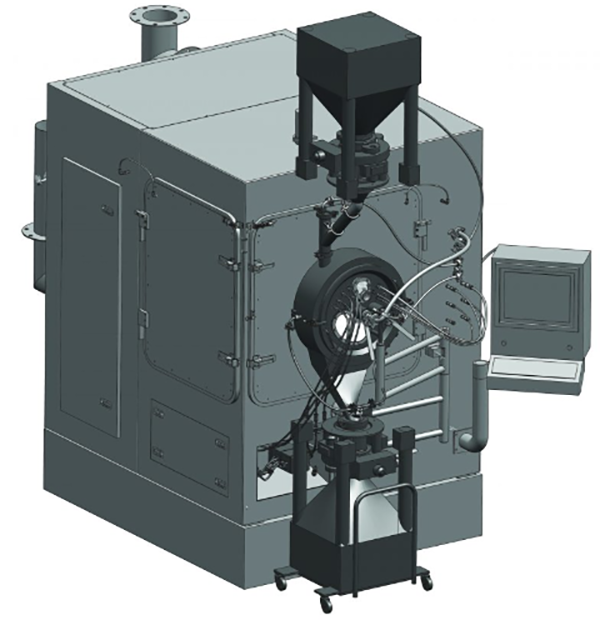
lConcept of Cleaning levels in Containment:
The cleaning process should guarantee the containment of all materials before accessing the system. Inside the isolator, spray nozzles are strategically positioned to clean 95% of the equipment, while wash wands can reach behind shadowed areas. The collected water is then treated separately. While basic cleaning requirements are straightforward to enact, they may fall short for larger facilities and more stringent standards. Cleaning procedures typically involve one of three methods:
Wet-In-Place (WIP): A frequently employed technique aimed at expediting the cleaning procedure. It involves saturating and dislodging surface debris before proceeding with thorough cleaning. Additionally, WIP serves to moisten components that may have been exposed to powders, mitigating the potential for airborne dust during equipment removal for cleaning.

Wash-In-Place (WIP): Denotes a cleaning process that is either semi or fully automated. The outcome of this process may be unclear in terms of meeting GMP (Good Manufacturing Practice) requirements or may result in a system that has not yet attained the desired level of cleanliness according to GMP standards.

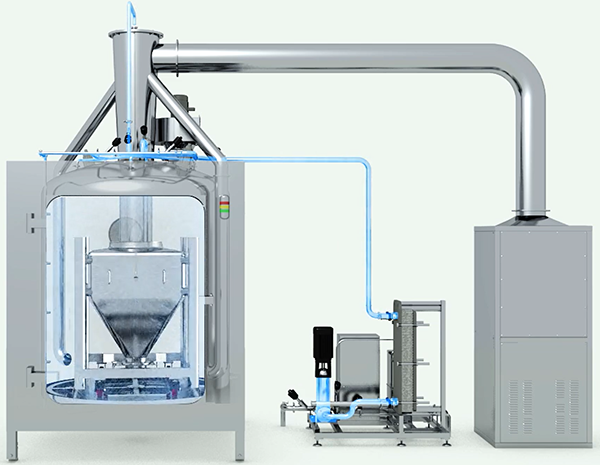
Clean-In-Place (CIP)involves a series of protocols implemented to thoroughly clean sections or the entirety of a processing system while it remains in position, without necessitating the removal or disassembly of equipment. The advantages of CIP are:
High Pressure / Low Volume Skid:The system utilizes a high-pressure and low-volume skid setup.
Faster Cleaning with Higher Pressure:The heightened pressure employed ensures quicker cleaning procedures, enhancing efficiency.
Reduced Water Usage:The system conserves water by operating with lower volumes, eliminating the necessity for additional tanks.
Decreased Operating Costs:With lower water usage, operating expenses are reduced, contributing to cost savings.
Less Wastewater Produced:The minimized water usage results in a reduction of wastewater generated, aligning with sustainability goals and regulatory requirements.
Automatic Bin Washing Station:for cleaning of IBC bins.







 Search
Search 中文
中文





























